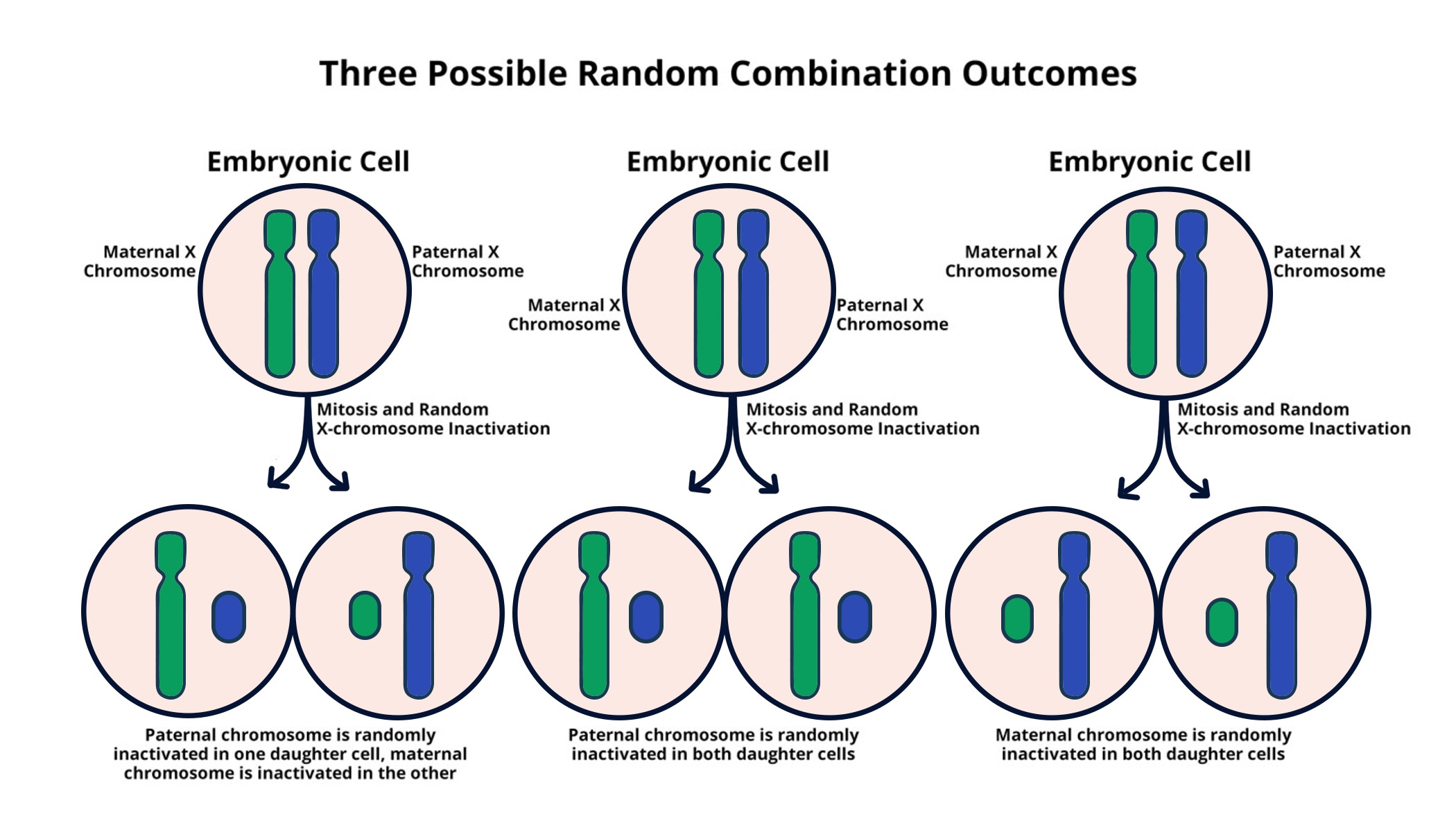
X-Chromosome Inactivation (XCI) is a fascinating biological phenomenon that plays a critical role in maintaining the balance of gene expression between males and females. In females, possessing two X chromosomes requires one to be silenced, ensuring that they do not produce excess levels of X-linked gene products. This intricate process is at the forefront of research, particularly in relation to disorders such as Fragile X Syndrome and Rett Syndrome, both linked to mutations on the X chromosome. Jeannie Lee’s groundbreaking work in this area has unveiled how specialized RNA molecules orchestrate the silencing of the X chromosome, leading to potential new therapies through gene therapy. As scientists continue to unravel the complexities of XCI, the possibilities for treating genetically based conditions look promising, heralding a new era in genetic research and therapeutic intervention.
The process of chromosomal silencing, specifically regarding X-Chromosome Inactivation (XCI), highlights an essential mechanism that distinguishes females from males in genetic expression. Females possess two copies of the X chromosome, making the need for one to be inactivated a crucial aspect of cellular function. This regulatory mechanism is not only vital for normal development but also directly influences disorders involving X-linked genes, such as Fragile X and Rett syndromes. Pioneering studies led by researchers like Jeannie Lee have shed light on this chromosomal phenomenon, revealing how specific molecular interactions mediate gene silencing. The insights gained from understanding XCI open new avenues for gene therapy, offering hope for innovative treatments for those affected by these genetic disorders.
The Role of X-Chromosome Inactivation in Genetic Disorders
X-Chromosome Inactivation (XCI) plays a crucial role in balancing gene dosage between males and females, who have distinct chromosomal configurations. Understanding how this process works is essential for addressing genetic disorders linked to the X chromosome, particularly Fragile X Syndrome and Rett Syndrome. In females, one X chromosome is inactivated early in development, which ensures that the overall expression of X-linked genes is similar to that of males, who possess only one copy. This intricate biological mechanism has drawn the attention of researchers worldwide, especially those aiming to alleviate conditions caused by mutations on the X chromosome.
Jeannie Lee’s pioneering research at Harvard Medical School sheds light on the molecular underpinnings of X-chromosome inactivation. By investigating how the gene Xist triggers changes in the chromosomal environment, scientists can better understand the silencing process that defines this critical phenomenon. The potential applications of their findings are vast, as restoring function to genes that were previously inactivated could lead to innovative treatments for genetic disorders like Fragile X Syndrome, which is particularly prevalent in males.
Jeannie Lee’s Contributions to Chromosomal Research
Jeannie Lee’s groundbreaking work delves into the intricate mechanisms of X-chromosome inactivation, focusing on the role of gel-like substances that facilitate this essential biological process. Her lab has made significant strides in comprehending how specific RNA molecules, such as Xist, manipulate the physical properties of the chromosomal environment, effectively silencing one X chromosome in females. This chromosomal breakthrough not only enhances our understanding of cell biology but also opens up new avenues for genetic research that could lead to viable therapeutic options for serious conditions linked to X-chromosome mutations.
By exploring the interactions between Xist and the gelatinous substance surrounding chromosomes, Lee and her team have the potential to influence the treatment landscape for conditions like Rett Syndrome. Their findings offer hope for innovative gene therapies aimed at activating the healthy genes in the inactivated X chromosome, providing pathways to treatment not previously thought possible. Such advancements signify a dramatic shift towards molecular medicine, as these insights pave the way for clinical trials and tailored therapies that could transform the quality of life for countless individuals afflicted by X-linked disorders.
Therapeutic Implications of X-Chromosome Inactivation Research
The implications of Jeannie Lee’s research on X-chromosome inactivation extend far beyond basic science. By unlocking the mechanisms behind XCI, her team is working to develop gene therapies that target diseases such as Fragile X Syndrome and Rett Syndrome. These genetic disorders result from mutations on the X chromosome, creating a need for precise interventions that can alleviate the symptoms or even cure the conditions altogether. Enhancing our understanding of how to ‘unsilence’ inactivated X chromosomes could provide a blueprint for designing targeted therapies.
Current research indicates that by freeing the functional genes trapped within the inactivated X chromosome, significant therapeutic effects could be achieved. Lee’s lab is actively developing methodologies to restore gene function, making it a beacon of hope in the field of genetic medicine. With the knowledge gained from this research, it may be possible to create treatments that not only aid females who carry mutations on one X chromosome but also benefit males affected by similar conditions. The ongoing research thus represents a crucial step towards personalized medicine where treatments are tailored to the unique genetic profiles of individuals.
The Intersection of Chromosomal Breakthroughs and Gene Therapy
The recent advances in understanding X-chromosome inactivation coincide with a broader surge in gene therapy techniques. These breakthroughs are pivotal in addressing genetic disorders that have long been thought to resist treatment. The intricate relationship between chromosomal behavior and therapeutic applications highlights the potential for innovative strategies to target specific genetic anomalies. Jeannie Lee’s ongoing studies not only unravel the mysteries of XCI but also demonstrate how these fundamental biological processes can be harnessed for designing effective gene therapies.
As preparations for clinical trials commence, the excitement surrounding these chromosomal breakthroughs is palpable. The ability to manipulate gene expression linked to X-linked disorders could revolutionize how such genetic diseases are treated. Lee’s research illustrates the potential of combining deep scientific inquiry with practical application, signalling a shift towards a new era of medical interventions that may soon offer relief to individuals suffering from Fragile X Syndrome and Rett Syndrome. This fusion of chromosomal studies and therapeutic innovation embodies the promise of modern genetics.
Understanding Fragile X Syndrome Through Chromosomal Research
Fragile X Syndrome is one of the most common hereditary causes of intellectual disability and is intricately linked to the dynamics of the X chromosome. By studying how X-chromosome inactivation affects gene expression, researchers like Jeannie Lee are unraveling the complexities of this syndrome. The fragility of the X chromosome, which can be exacerbated by environmental factors and gene mutations, creates unique challenges for affected individuals. By identifying the mechanisms behind XCI, significant strides can be made in the treatment and management of this condition.
Lee’s research not only provides insight into the biological basis of Fragile X Syndrome but also lays the groundwork for potential therapeutic interventions. The ability to reactivate silenced genes on the X chromosome could offer hope for restoring normal gene function in those affected by the syndrome. With the application of gene therapy techniques, the opportunity to alleviate the symptoms and improve outcomes for individuals diagnosed with Fragile X Syndrome shines on the horizon, changing lives through targeted molecular medicine.
Rett Syndrome: Implications of Gene Therapy Solutions
Rett Syndrome is another debilitating neurodevelopmental disorder associated with mutations on the X chromosome, primarily affecting females. Understanding the mechanisms behind X-chromosome inactivation is vital in addressing diseases like Rett Syndrome, as selective gene silencing can lead to severe developmental challenges. Jeannie Lee’s work opens new prospects for gene therapy aimed at restoring function to mutated genes associated with Rett Syndrome. It emphasizes the need to unearth the potential residing within seemingly inactive genetic material.
With the research realities converging at the frontier of gene therapy, scientists have a clearer path toward potential treatments for Rett Syndrome. Lee’s innovative approaches to unlocking silenced genes could usher in new therapeutic options, significantly changing the prognosis for affected individuals. These advancements point to a future where gene therapy could not only mitigate the symptoms of Rett Syndrome but potentially restore neurological function, offering hope to families worldwide.
The Future of Gene Therapy: Toward Clinical Trials
As Jeannie Lee’s team prepares to transition from laboratory research to clinical trials, the vision for gene therapy becomes increasingly tangible. The implications of their work in X-chromosome inactivation open doors to novel treatment strategies for various X-linked disorders such as Fragile X Syndrome and Rett Syndrome. The upcoming trials will be pivotal in validating the efficacy of these therapies and addressing crucial safety concerns, ultimately moving closer to the clinic and making these advanced treatments accessible to those in need.
The future of gene therapy resides in the convergence of rigorous scientific research and clinical application. Lee’s focus on optimizing therapeutic approaches reflects a broader trend towards personalized medicine, where treatments can be tailored to the genetic makeup of individuals. The excitement surrounding these developments fuels anticipation for the upcoming trials and the hope that these groundbreaking findings will translate into real-world solutions, heralding a new era in the treatment of genetic disorders.
Challenges in X-Chromosome Research and Beyond
Despite the significant advancements brought forth by Jeannie Lee’s research, challenges remain in fully understanding the complexities of X-chromosome inactivation. The process itself is multifaceted, involving various molecular components and regulatory systems that require further elucidation. While the breakthroughs in chromosomal and gene therapy are promising, researchers must also navigate the intricacies of gene regulation and expression to ensure that new therapies are both effective and safe for clinical use.
Addressing such challenges necessitates a multidisciplinary approach, wherein collaboration among geneticists, molecular biologists, and clinicians becomes essential. As the field progresses, expanding our understanding of X-chromosome dynamics will pave the way for other chromosomal therapies. This interconnected approach will play a vital role in transforming fundamental genomic discoveries into practical applications, enhancing our ability to combat a range of genetic disorders effectively.
Long-Term Perspectives on Chromosomal Adjustments
The long-term implications of advancements in our understanding of X-chromosome inactivation may extend beyond the immediate applications in Fragile X Syndrome and Rett Syndrome. The principles and methodologies developed through this research could have wide-ranging effects on the treatment of other genetic disorders linked to distinct chromosomal arrangements. As scientists continue to unravel the complexities of XCI, our knowledge of chromosomal genomics and its applications in therapeutic contexts is likely to expand.
Moreover, as we gather more insights into how to manipulate chromosomal processes, there arises a profound ethical question regarding the application of such technologies. Society must engage in discussions about the balance between potential benefits and the responsibility of altering genetic material. By approaching these developments with a comprehensive understanding and an ethical framework, we can navigate the future of gene therapy and chromosomal research responsibly and thoughtfully.
Frequently Asked Questions
What is X-Chromosome Inactivation and why is it important in understanding disorders like Fragile X Syndrome?
X-Chromosome Inactivation (XCI) is a crucial biological process whereby one of the two X chromosomes in females is silenced to ensure dosage compensation between males and females. This inactivation is particularly important when studying disorders like Fragile X Syndrome, which is caused by mutations on the X chromosome. By understanding XCI, researchers can explore potential treatments, such as gene therapy, that could reactivate healthy genes on the inactive X chromosome, thus providing therapeutic options for affected individuals.
How does Jeannie Lee’s research contribute to the understanding of X-Chromosome Inactivation and its effects on Rett Syndrome?
Jeannie Lee’s research has significantly advanced our understanding of the mechanisms behind X-Chromosome Inactivation. Her lab discovered the role of the RNA molecule Xist in orchestrating this process, which is critical in understanding conditions like Rett Syndrome, another X-linked disorder. By investigating how this inactivation affects gene expression, Lee’s work lays the groundwork for potential therapies that may alleviate symptoms of Rett Syndrome by targeting the silenced X chromosome.
What role does the ‘gelatinous substance’ mentioned in the research play in X-Chromosome Inactivation?
The ‘gelatinous substance’ surrounding chromosomes acts as a structural component that facilitates X-Chromosome Inactivation. This substance changes physical properties when engaged with the RNA molecule Xist, allowing for the effective silencing of one X chromosome in females. Understanding this mechanism is crucial as it could lead to breakthroughs in gene therapy for X-linked disorders like Fragile X Syndrome and Rett Syndrome.
Can X-Chromosome Inactivation be used to develop gene therapies for diseases like Fragile X Syndrome?
Yes, breakthroughs in understanding X-Chromosome Inactivation, particularly work by Jeannie Lee, suggest that it may be possible to develop gene therapies that reactivate the healthy gene located on the inactive X chromosome. This approach could offer new treatment options for individuals with Fragile X Syndrome, allowing them to utilize the functional copy of the gene needed to mitigate symptoms.
What implications does the discovery of mechanisms behind X-Chromosome Inactivation have for male patients with X-linked disorders?
The discovery of mechanisms behind X-Chromosome Inactivation has important implications for male patients as well. While males have a single X chromosome, understanding how genes are silenced on this chromosome can lead to strategies that unmask mutated genes, like those involved in Fragile X Syndrome. Hence, insights from XCI research could open up new avenues for treatment even for males affected by X-linked disorders.
How could freeing inactivated X chromosomes lead to a cure for conditions like Fragile X Syndrome and Rett Syndrome?
Freeing inactivated X chromosomes could potentially cure conditions like Fragile X Syndrome and Rett Syndrome by allowing access to healthy gene copies that were previously silenced. Researchers, including Jeannie Lee, are exploring therapies to unsilence X-linked genes, which could restore normal gene function and alleviate the symptoms associated with these genetic disorders.
| Key Point | Details |
|---|---|
| X-Chromosome Challenge | Females have two X chromosomes, requiring inactivation of one to balance gene dosage with males, who have one X chromosome. |
| Role of Xist Gene | Xist RNA interacts with a gelatinous substance around the X chromosome, aiding in its inactivation. |
| Gelatinous Substance Function | This substance keeps chromosomes separate, preventing them from tangling and allowing Xist to modify its properties. |
| Therapeutic Potential | Unlocking the inactivated X chromosome could provide treatment options for genetic disorders like Fragile X and Rett syndrome. |
| Future Research Directions | Ongoing studies aim to optimize unsilencing methods and prepare for clinical trials. |
Summary
X-Chromosome Inactivation is a crucial biological process that ensures females with two X chromosomes do not express double the amount of X-linked genes compared to males. Recent research by Jeannie T. Lee has unveiled how this complex process works and its potential implications for treating genetic disorders linked to the X chromosome. The findings indicate that the Jell-O-like substance around chromosomes plays a vital role, enabling the Xist gene to modify its characteristics for effective inactivation. This pathway opens exciting avenues for therapies that could transform the treatment landscape for conditions such as Fragile X Syndrome and Rett Syndrome, making significant strides toward potential clinical applications.